Introduction
One of the most common product safety related analytical tests is the quantification of inorganic impurities within a pharmaceutical product. This includes toxic heavy metals, such as As, Cd, Hg, and Pb. Other metals, such as Fe, Cr, Ni and Zn, are also of interest due to health risks. In addition, many Active Pharmaceutical Ingredients (APIs) may contain residual metal catalysts, such as Ru, Pt, and Pd. Since there are many potential sources of contamination, it may be of interest to measure raw materials, intermediates as well as final products. This study demonstrates that the X-ray Fluorescence (XRF) technique is capable of performing elemental analysis of all of these pharmaceutical (liquid, powder and solid) materials with high sensitivity, precision and accuracy. Simple sample preparation, non-destructive analysis, a wide dynamic range and good to excellent detection limits across large parts of the periodic table are some of the advantages of this method.
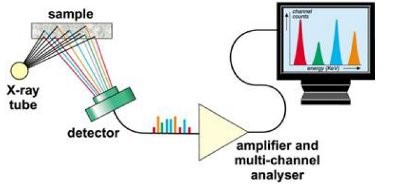
X-ray Fluorescence (XRF) spectrometry
XRF (X-ray fluorescence spectrometry) is a non-destructive analytical technique used to identify and determine the concentrations of elements present in solid, powdered and liquid samples. XRF is capable of measuring elements from beryllium (Be) to uranium (U) and beyond at trace levels often below one part per million and up to 100%. The XRF spectrometer measures the individual component wavelengths of the fluorescent emission produced by a sample when irradiated with X-rays.
XRF instrumentation is conventionally divided into Wavelength Dispersive (WDXRF) and Energy Dispersive (EDXRF) instruments, the distinguishing factor being the technologies used for the energy discrimination and detection of the X-ray photons. WDXRF instrumentation typically has much higher power loading on the sample than EDXRF. This may limit the use of the WDXRF technique (typically with power in kW range) for organic matrix sample analysis because the heat generated by the X-rays may be sufficient enough to induce sample alteration, such as browning or discoloring of the sample surface or loss of volatile elements (e.g. Hg and Se). EDXRF systems, on the other hand, use low power X-ray sources or secondary targets and will not significantly heat the sample. As the bulk of pharmaceutical materials are organic in nature the focus of this white paper is to emphasize the usefulness of the EDXRF technique, which has the best potential for the measurement of all elements from Na - U in pharmacological-type samples.
The detector system in EDXRF determines the energy of the X-ray photons and accumulates the data in a multichannel analyzer. The resulting spectrum is used in subsequent steps to extract information regarding the elemental composition of the specimen. Essentially, EDXRF is a simultaneous technique as it accumulates information about all elements in the sample at the same time. The performance can be significantly boosted by optimizing the excitation conditions for the elemental ranges of interest. Two or more spectra (each obtained with different measurement conditions) are then obtained. Calibration procedures for quantification are carried out through normal spectroscopic techniques involving the measurement of standards of varying elemental concentrations. Blanks may be included but these are not essential. XRF calibrations have the advantage over other elemental analysis methods, such as Inductively Coupled Plasma (ICP) or Atomic Absorption (AA) spectroscopy, of being long lasting, often more than a year before any recalibration is required
Matrix correction
In XRF either undiluted samples or samples with a low dilution are measured. This results in matrix effects: the presence of one element that influences the measured intensity of the others. At elevated levels one element might (through processes such as absorption or enhancement) affect the intensity of another significantly. These matrix effects are well understood in XRF, and several methods have been developed to deal with them. The European Pharmacopeia lists the use of the Compton method to correct for matrix effects when analyzing trace levels. The Compton correction method is a straight-forward and effective method for such applications. It consists of taking the ratio of the measured intensity of the analyte and the intensity of a scattered line which is representative of the excitation. This scattered line is a characteristic line from either the X-ray tube of from the secondary target used. The Compton correction method corrects for matrix effects, and also to some extent for variations in sample packing when loose powders are analyzed.
Drift correction
The intensity of an X-ray instrument gradually deteriorates with time in a rather predictable fashion. Measuring this change in intensity with time allows for the determination of a drift factor, which can be applied to the calibration curves to keep them accurate over long periods of time. Most manufacturers provide drift correction routines with the software. High-end X-ray instrumentation, with specifically designed tubes is very reliable and stable; in some cases of the drift needs only to be corrected for once a month. The calibration curves can be used for much longer periods of time.
Experiment
This white paper focuses on the analysis of excipient materials doped with known concentrations of metals. Standard samples were produced using fine excipient grade cellulose and pure organo-metallic compounds. ICP-MS techniques were employed to independently confirm the concentrations of elements in each standard. These standards were prepared and measured as both loose powders and pressed pellets. All loose powder standards and samples were weighed into disposable sample cups with 4.2 micrometer polypropylene support film. Compton ratio corrections were employed to account for any minor variations in sample packing. All pressed pellets were produced without a binder and pressed for 60 seconds at 10 tons.
These materials (standards) were used to set up calibrations on a SPECTROXEPOS EDXRF instrument. The SPECTROXEPOS instrument employs a tunable excitation strategy which includes multiple secondary targets. In additional it has a polarized optical path which results in low backgrounds.
Conclusions:
The EDXRF technique is a robust, precise, sensitive and accurate method with the potential to analyze inorganic impurities in many types of pharmaceutical materials. The analysis of the elements Al, Sb, As, Cd, Cr, Co, Cu, In, Ir, Fe, Hg, Pb, Mg, Mn, Hg, Mo, Ni, Os, Pd, Pt, Rh, Ru, Ru, Se, Sr, Tl, Sn, W and Zn are all feasible by EDXRF. The EDXRF technique is capable of generating reporting limits that comply with current and proposed pharmacopeia limits for these metals.
The simplified sample preparation technique which included direct liquid analysis without the concern of salt concentrations or acidity levels, direct loose powder or press pellet analysis, presents a great advantage over other analytical techniques. This represents a significant simplification in the analysis of pharmaceutical materials.






